Basic and Translational Research
- Sushil K. Mahata
- Robert Parmer
- Prabhleen Singh
- Scott C. Thomson
- Volker Vallon
- Michael G. Ziegler
Obesity plays a crucial role in the development of insulin resistance in humans worldwide. Obesity induces low-grade tissue inflammation, which leads to insulin resistance and the consequent development of type 2 diabetes mellitus. Cells of the innate immune system (macrophages, neutrophils and natural killer cells) produce inflammatory cytokines and other factors that impair insulin signaling. Adaptive immune cells (CD8+ effector T and adipose tissue B2 cells) also contribute to the development of obesity-induced insulin resistance. The Mahata lab has recently shown that an endogenous chromogranin A (CgA) peptide catestatin (CST: hCgA352-372) inhibits the recruitment of monocyte-derived macrophages to the liver and decreased inflammation by decreasing proinflammatory cytokines in the blood, which suggests that CST acts as an anti-inflammatory peptide. In addition, CST directly suppresses glucose production from hepatocytes and indirectly suppresses lipid accumulation in liver. Therefore, one of the major focus of the Mahata lab is to gain insight into the mechanisms underlying CST and its regulation of diet-induced inflammation and insulin resistance with an emphasis on the involvement of innate and adaptive immune cells.
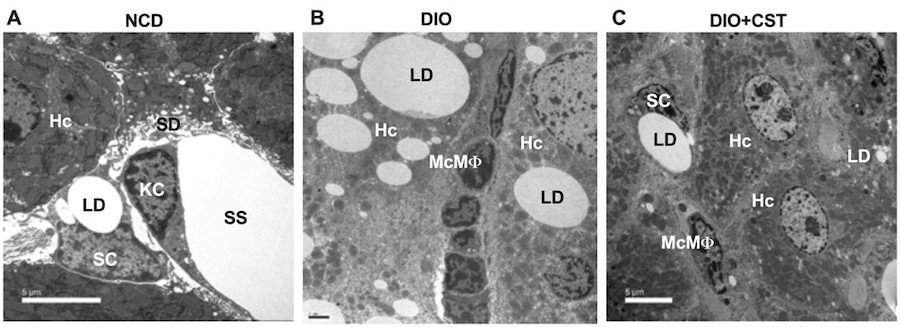
Figure 1. Transmission Electron Micrographs of liver sections. (A) Wild-type liver showing hepatocytes (Hc), stellate cell (SC) and Kupffer cell (KC). (B) Diet-induced obese (DIO) liver showing infiltration of monocyte-derived macrophages (McMf). (C) CST-treated DIO liver showing marked inhibition of infiltration of McMf. LD, lipid droplet; SD, space of Disse; SS, sinusoidal space (Diabetes 67: 841-848, 2018).
While innate immune cells in the resting heart include macrophages, neutrophils and mast cells, adaptive immune cells include B cells and regulatory T cells (TReg). Cells of the innate and adaptive immune system contribute to the development of hypertension. The loss of Ly6Chigh monocytes prevents hypertension-induced cardiac fibrosis and improves cardiac function after myocardial infarction. Preclinical and genome-wide association studies suggest a positive role for both CD8+ and CD4+ T cells in the development of human hypertension. Based on the anti-inflammatory effects of CST, the second major focus of the Mahata lab is to explore the contribution of innate and adaptive immune cells in executing the beneficial effects of CST on cardiovascular diseases.
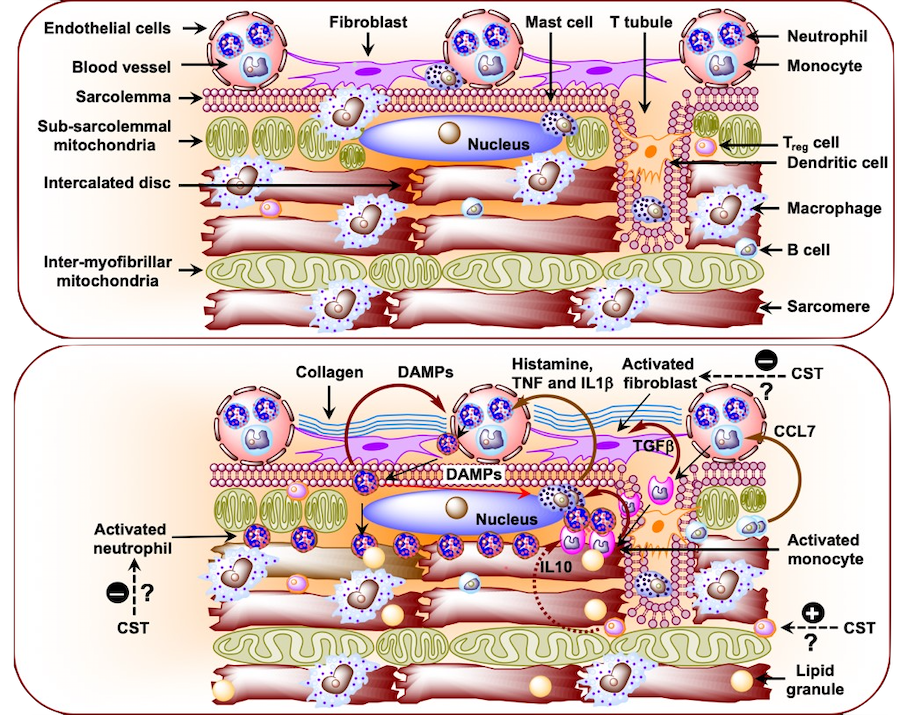
Figure 2. (A) Schematic diagram showing immune cells in the healthy heart. (B) Schematic diagram showing infiltration of neutrophils and monocytes from the circulation and their effects on resident immune cells in the diabetic cardiomyopathy heart. CCL7, chemokine (C-C motif) ligand 7; DMPP, damage-associated molecular pattern; IL1b, interleukin 1b; TNF, tumor necrosis factor; TGFb, transforming growth factor beta (Curr Med Chem 25: 1352-1374, 2018).
To address the above questions, the Mahata lab uses diet-induced and genetically engineered obese mice as well as mice with systemic and tissue-specific knockout of the CgA gene and specifically the CST domain. The ongoing studies utilize biochemical assays (glycogen, lipids and proteins), FACS analysis, irradiation and bone-marrow transplantation, RNA Sequence analysis, and ultrastructural analyses to assess the involvement of CST and immune cells in obesity-induced insulin resistance and diabetes as well as genetically-engineered hypertension.